Poster Presentation 33rd ASM of the Australian & New Zealand Bone & Mineral Society 2023
Concurrent denosumab and parenteral iron therapy precipitating severe hypocalcaemia and hypophopshataemia: a case report (#258)
Background
Parental iron and denosumab are commonly prescribed drugs within inpatient and community settings. There is increasing awareness of possible drug-drug interactions and risk of FGF23 mediated hypophosphataemia4, which can be severe and life-threatening4,5. Close monitoring and prompt treatment of electrolyte disturbance is required, whilst avoiding significant delays in denosumab treatment.
Case
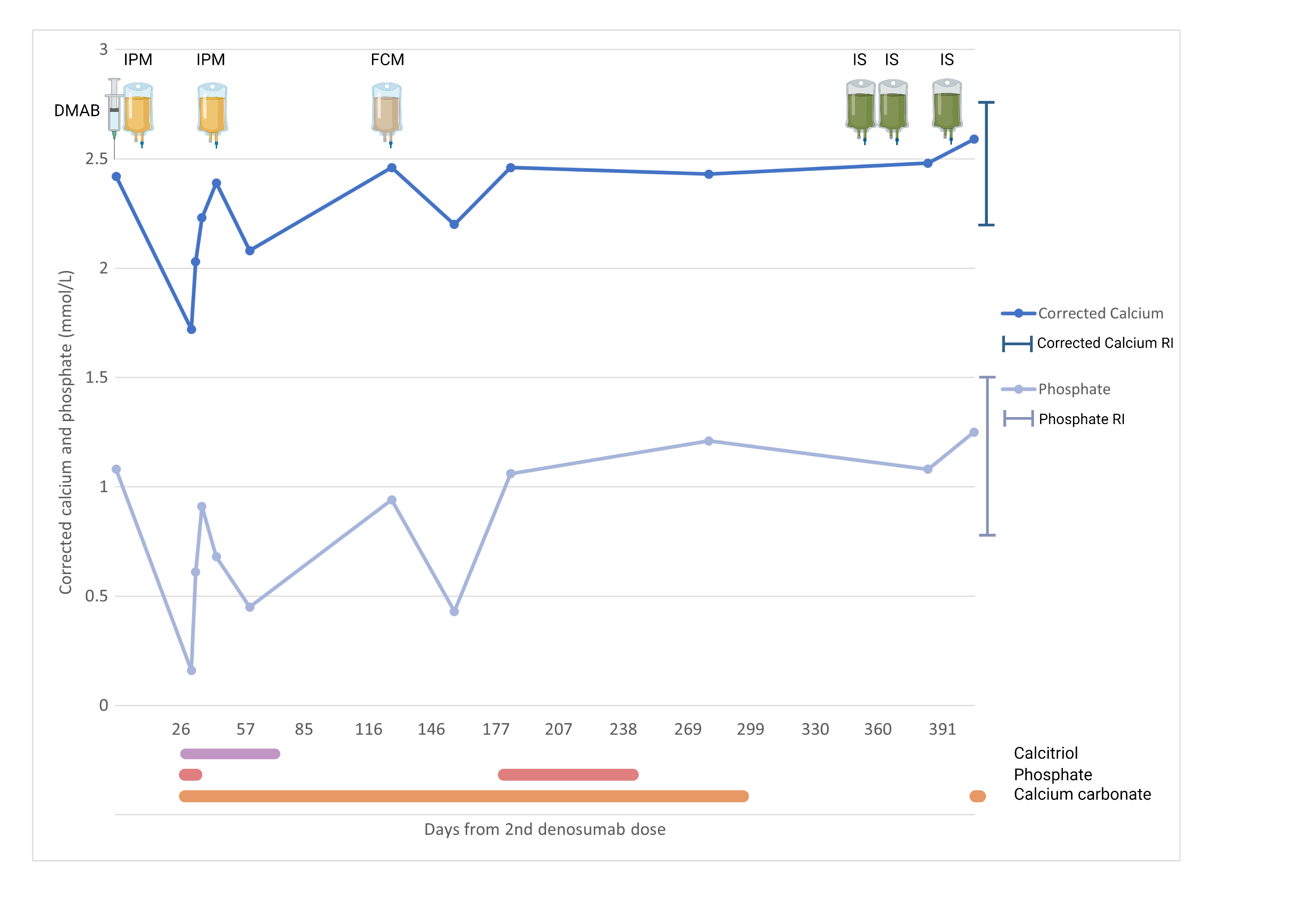
A 76-year-old man presented to the Emergency Department with lethargy and mixed respiratory failure. He was found to have severe hypocalcaemia (1.72mmol/L; reference range [RR]: 2.15-2.65), hypophosphataemia (<0.16mmol/L; RR: 0.75-1.50) and QT prolongation (525ms) on electrocardiogram. He had normal creatinine (86micromol/L; RR: 60-110), hyperparathyroidism (36.9pmol/L; RR: 2.0-8.5) and sufficient 25-OH vitamin D levels (67nmol/L; RR: >50).
Four weeks prior, he had received his second dose of denosumab 60mg for a diagnosis of osteoporosis (hip T-score -2.6; spine T-score -1.6). Two weeks later he was also administered intravenous iron polymaltose (Figure 1) for chronic iron deficiency anaemia secondary to gastrointestinal angioectasiae, requiring frequent intravenous iron infusions every 3 months for the last 12 months.
The patient required urgent treatment with non-invasive ventilation, cardiac telemetry, and intravenous calcium gluconate and phosphate replacement. Oral calcitriol, calcium carbonate and phosphate were commenced once his electrolytes stabilised, and gradually weaned following discharge
Despite multiple treatments with argon plasma coagulation of angioectatic lesions, he required further iron infusions with iron polymaltose and ferric carboxymaltose. Each was complicated by transient hypophosphataemia without hypocalcaemia, requiring calcitriol and phosphate replacement (nadir phosphate levels 0.68mmol/L and 0.43mmol/L, respectively). Subsequent investigations at the time of recurrent hypophosphataemia demonstrated hyperparathyroidism ((24.7pmol/L), normal 25-OH vitamin D (102nmol/L), and an inappropriately high fractional excretion of phosphate (36%; RR: 10-20%). Fibroblast growth factor 23 (FGF23) levels were not available at the time of the hypophosphataemia.
A diagnosis of recurrent iron infusion-related (FGF23-mediated) hypophosphataemia, and a subsequent denosumab and iron infusion interaction causing a presentation with profound hypocalcaemia and hypophosphataemia was made. Denosumab was ceased. Unfortunately, persistent iron deficiency anaemia necessitated further parental iron therapy. Iron sucrose has since been administered on multiple occasions without calcium or phosphate disturbance.
Discussion
According to Pharmaceutical Benefits Scheme (PBS) data, parenteral iron administration has rapidly increased from ~70,000 prescriptions in 2014 to more than 160,000 in 2016, with ferric carboxymaltose most frequently prescribed following its PBS listing1. With increasing indications1, parenteral iron is prescribed by a range of clinicians, including general practitioners, gastroenterologists, nephrologists, haematologists and internal medicine physicians1. Denosumab is now the most frequently administered osteoporosis therapy, rising from 36.5% to 76.1% of prescribed osteoporosis treatments between 2014-20182. Denosumab induced hypocalcaemia is well recognised, in dialysis and advanced chronic kidney disease patients, and in the setting of vitamin D deficiency. Recent reports have highlighted the problem of FGF23 mediated hypophosphataemia secondary to iron infusions, and iron infusion and denosumab drug-drug interactions are now starting to be reported3-6.
The mechanism of hypocalcaemia and hypophosphataemia with intravenous iron and denosumab has been proposed, relating to regulation via PTH, 1,25-(OH)2 vitamin D and FGF23. Denosumab inhibits osteoclastic bone resorption, transiently reducing plasma calcium concentration and resulting in elevated PTH levels7. The subsequent hyperparathyroidism decreases renal phosphate reabsorption and promotes phosphaturia. Iron infusions inhibit cleavage and inactivation of FGF238. High levels of FGF23 decrease renal phosphate reabsorption and inhibit renal 1α-hydroxylation of 25-OH vitamin D to activated 1,25-(OH)2 vitamin D, thereby impairing intestinal absorption of calcium and phosphate4. Recurrent iron infusion-related hypophosphataemia has been associated with osteomalacia. Co-administration of iron infusions and denosumab may compound the phosphaturia. Additionally, FGF23 mediated impairment of 1,25-(OH)2 vitamin D activation blunts the physiological response to hypocalcaemia induced by denosumab, further contributing to both hypocalcaemia and hypophosphataemia. Acute hypophosphataemia and hypocalcaemia can cause tetany, cardiorespiratory failure, seizures and coma in severe cases7,8.
A strategy to safely administer iron infusions in patients on denosumab is necessary, due to risk of rebound bone loss and vertebral fractures with delay of denosumab. Although hypophosphataemia was observed in our case with both iron polymaltose and ferric carboxymaltose, hypophosphataemia was not observed upon challenging with iron sucrose. This may be due to the waning effect of denosumab, or may support the proposal that different iron preparations may have varying risk of hypophosphataemia9,10. From meta-analysis data, ferric carboxymaltose was associated with a significantly higher risk of hypophosphataemia than iron isomaltose (risk ratio 7.90, 95% confidence interval 2.10-28.0) and iron sucrose (risk ratio 9.40, 95% confidence interval 2.30-33.0)9. Electrolyte disturbance occurred in cases at 8-26 days post denosumab3-6, so reasonable monitoring could include at 7, 14 and 28 days and not thereafter if there are no abnormalities. Studies to determine the frequency of this serious drug interaction, and to assess which iron preparations reliably reduce the risk of hypophosphatemia with denosumab are required.
Learning points
- Denosumab and parenteral iron replacement have increasingly been used after PBS listing and are prescribed across multiple specialties and settings.
- Although denosumab induced hypocalcaemia and iron infusion related hypophosphataemia are well described, this case highlights that co-prescription increases the risk of life-threatening electrolyte derangement requiring hospital admission and parenteral therapy.
- Clinicians should be alert to the potential drug interaction between denosumab and iron infusions, in order to develop strategies to prevent, monitor and mitigate the electrolyte abnormalities.
- Alternative iron preparations may be associated with a reduced risk, however further research to determine the frequency of the interaction and reliability of different parenteral iron formations in reducing risk of hypophosphataemia is required.
Figure 1 – Timeline of serum correct calcium and phosphate in relation to denosumab, iron infusions (iron polymaltose, ferric carboxymaltose, iron isomaltose)
- 1. Department of Health. Ferric carboxymaltose: 24 month predicted versus actual analysis. Canberra: Department of Health; 2017. Available from: https://www.pbs.gov.au/info/industry/listing/participants/public-release-docs/dusc-public-release-documents-by-condition (accessed Mar 2023).
- 2. Department of Health. Denosumab for osteoporosis: utilisation analysis using PBS data. Canberra: Department of Health; 2020. Available from: https://www.pbs.gov.au/info/industry/listing/participants/public-release-docs/dusc-public-release-documents-by-condition (accessed Mar 2023).
- 3. Cohen A, Chacko B. Severe hypocalcaemia following denosumab and iron infusion. Nephrology. 2022;27(9):781-2. Doi:https://doi.org/10.1111/nep.14078.
- 4. Smyth B, Ong S. Severe hypocalcaemia and hypophosphataemia following intravenous iron and denosumab: a novel drug interaction. Intern Med J. 2016 Mar;46(3):360-3. doi: 10.1111/imj.13001. PMID: 26968599.
- 5. Fang W, McMahon LP, Bloom S, Garg M. Symptomatic severe hypophosphatemia after intravenous ferric carboxymaltose. JGH Open. 2019;3(5):438-40. Doi:10.1002/jgh3.12150.
- 6. Tai R, Mouchaileh N, Ting C. Hypocalcaemia and hypophosphataemia following denosumab and IV ferric carboxymaltose in an older patient with normal renal function. Journal of Pharmacy Practice and Research. 2022;52(1):49-52. Doi:https://doi.org/10.1002/jppr.1791.
- 7. Megapanou E, Florentin M, Milionis H, Elisaf M, Liamis G. Drug-Induced Hypophosphatemia: Current Insights. Drug Saf. 2020 Mar;43(3):197-210. doi: 10.1007/s40264-019-00888-1. PMID: 31776845.
- 8. Coppolino G, Nicotera R, Cernaro V, Calimeri S, Leonardi G, Cosentino S, Comi A, Donato C, Lucia CM, Provenzano M, Michael A, Andreucci M. Iron Infusion and Induced Hypophosphatemia: The Role of Fibroblast Growth Factor-23. Ther Apher Dial. 2020 Jun;24(3):258-264. doi: 10.1111/1744-9987.13435. Epub 2019 Oct 25. PMID: 31483921.
- 9. Bellos I, Frountzas M, Pergialiotis V. Comparative Risk of Hypophosphatemia Following the Administration of Intravenous Iron Formulations: A Network Meta-Analysis. Transfus Med Rev. 2020 Jul;34(3):188-194. doi: 10.1016/j.tmrv.2020.07.002. Epub 2020 Jul 23. PMID: 32819760.
- 10. Schaefer B, Tobiasch M, Viveiros A, Tilg H, Kennedy NA, Wolf M, Zoller H. Hypophosphataemia after treatment of iron deficiency with intravenous ferric carboxymaltose or iron isomaltoside-a systematic review and meta-analysis. Br J Clin Pharmacol. 2021 May;87(5):2256-2273. doi: 10.1111/bcp.14643. Epub 2020 Dec 7. PMID: 33188534; PMCID: PMC8247006.