Oral Presentation 33rd ASM of the Australian & New Zealand Bone & Mineral Society 2023
Severe osteoporosis secondary to systemic mastocytosis exacerbated by pregnancy and breastfeeding (#26)
Case summary
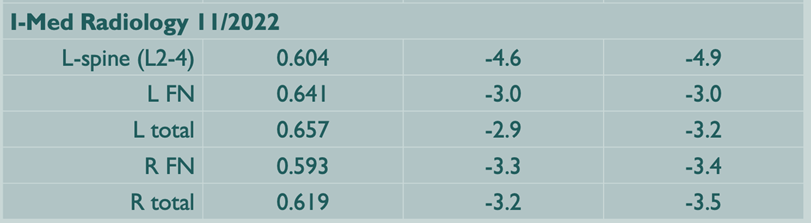
A 38-year-old woman was referred to endocrinology with an 8 month history of atraumatic lower back pain, beginning 2 months post-partum. An earlier lumbar spine MRI had revealed mild compression fractures of all lumbar superior endplates with bony edema indicating recency. DXA scan showed severe bone loss (L1-4 Z-scores all < -4.0 SD, Left and right femoral neck, -3.0 SD and -3.4 SD respectively.) There was no evidence of gastrointestinal, renal or endocrinological disorder. Family history revealed osteoporosis in her mother, aged in her 60s. She was advised to cease breastfeeding immediately, based on a presumptive diagnosis of pregnancy and lactation induced osteoporosis (PLO). As future pregnancy was being considered, anti-resorptive agents were not immediately commenced, hoping cessation of lactation might lead to improvement.
Subsequently, a further history of recent onset stress-induced vomiting, palpitations and flushing and prior episodes of angioedema to multiple analgesic agents and shellfish-induced anaphylaxis came to light. This prompted measurement of tryptase, which was elevated at 27.4 ug/L (N<11.4). Skin biopsy of discrete maculopapular lesions (Fig 1) present since her early twenties revealed mast cells >15 per high powered field. Bone marrow biopsy and flow cytometry revealed 100% of mast cells expressing CD2 and/or CD25 consistent with an abnormal phenotype. She was diagnosed with systemic mastocytosis and is undergoing subtype differentiation. She has lost 8cm in height in a year and her back pain remains debilitating, though imaging reveals no new fracture development. She commenced IV zoledronic acid, as her priority was to prevent further debilitating fractures, and pregnancy was now not desired in the next few years.
Background
Systemic mastocytosis (SM) is the pathological accumulation of mast cells in tissues and release of vasoactive substances such as histamine and prostaglandins, leading to symptoms and signs consistent with allergy or anaphylaxis. Of the subtypes, indolent systemic mastocytosis is the most common, accounting for more than 80% of all SM1.
There is a varying clinical spectrum of bony manifestations in SM, from bony pain, osteopenia, osteoporosis with fragility fractures, osteolytic lesions and osteosclerosis3. Osteoporosis is usually a feature of ISM, with increased BMD and osteosclerosis more common in the aggressive subtype4. The lumbar spine is most commonly involved, as mast cells tend to colonise the more metabolically active axial skeleton, rather than the fatty marrow in the appendicular skeleton5 . In a large German retrospective cohort of over 8000 patients with osteoporosis, the prevalence of ISM was 0.5%. However, in a subgroup of young male patients, the prevalence was more than 5%2.
The pathogenesis of osteoporosis in SM is complex but is thought to be due to the effect of inflammatory mediators that directly activate osteoclasts through the RANK-L system, leading to bone resorption6,7. However, not all patients with systemic mastocytosis develop osteoporosis (prevalence 18 – 31% in various studies), and increased BMD/osteosclerosis positively correlates with tryptase levels in the aggressive form. Other pathways may be involved, including the canonical Wnt-signalling pathway. Elevations in either DKK1 or sclerostin have been demonstrated in ISM versus control, though study results are somewhat conflicting8,9.
A previous Australian case report describes two women presenting with multiple thoracolumbar vertebral fractures in the lactation period. Both had worse spinal versus femoral neck BMD and were eventually diagnosed with systemic mastocytosis10. We postulate that in these cases, and ours, vertebral fractures resulted from the combined effect of lactation-associated bone resorption and SM on the axial skeleton.
Bisphosphonates are considered first line treatment for SM-induced osteoporosis. Small studies have shown improved BMD at the lumbar spine, reduced bone turnover and improved pain with bisphosphonate use5. However, no randomized placebo-controlled trials exist, to conclusively demonstrate fracture risk reduction. One study of bisphosphonate use in ISM-induced osteoporosis demonstrated ongoing fracture risk, with 5 and 10-year fracture free survival of only 81.9% and 67% respectively11. There are limited data on alternative therapies such as denosumab12, and no data on the use of anabolic agents. Teriparatide is said to potentially induce growth and proliferation of mast cells4, though mouse studies show teriparatide decreased mast cells numbers13.
Osteoporosis treatment in women of childbearing potential is a complex issue. No drug has proven safety in pregnancy. Animal studies suggest reproductive toxicity14,15, but small case series of bisphosphonate use prior to pregnancy have described non-specific complications without consistent signal for teratogenicity16,17. The extended half-life of bisphosphonates in bone is problematic, no guidelines exist on appropriate washout periods for different agents prior to pregnancy.
Take home messages
- Systemic mastocytosis is a rare but important differential to consider in younger patients with osteoporosis, presenting with vertebral fractures, without the typical risk factors for osteoporosis. This diagnosis should even be considered in the absence of allergy/anaphylaxis symptoms.
- Bisphosphonates are first-line in the management of SM-induced osteoporosis however there is limited data in its prevention of further fractures. The relative role of denosumab, teriparatide or romosozumab remains undetermined.
- Treatment decisions for premenopausal women at high risk of fracture requires an individualised approach, balancing morbidity from fracture with potential reproductive harm.
Figure 1: maculopapular lesions over trunk

Baseline blood tests Aug 2022

Further tests Dec 2022
0
MRI Spine Aug 2022

DXA Nov 2022

Bone marrow biopsy May 2023: IHC stain for CD 25

- 1. Cohen, S. S., Skovbo, S., Vestergaard, H., Kristensen, T., Møller, M., Bindslev-Jensen, C., Fryzek, J. P., & Broesby-Olsen, S. (2014). Epidemiology of systemic mastocytosis in Denmark. British journal of haematology, 166(4), 521–528.
- 2. Gehlen, M., Schmidt, N., Pfeifer, M., Balasingam, S., Schwarz-Eywill, M., Maier, A., Werner, M., & Siggelkow, H. (2021). Osteoporosis Caused by Systemic Mastocytosis: Prevalence in a Cohort of 8392 Patients with Osteoporosis. Calcified tissue international, 109(6), 685–695.
- 3. Greene LW, Asadipooya K, Corradi PF, Akin C. Endocrine manifestations of systemic mastocytosis in bone (2016). Rev Endocr Metab Disorder, 17(3):419–31.
- 4. Asadipooya, Kamyar & Greene, Loren. (2020). Chapter 8: Systemic Mastocytosis and Bone-Related Events. In Akim, C (Eds) Mastocytosis: A comprehensive guide (pp. 123-140) Springer Nature Switzerland
- 5. Rossini, M., Zanotti, R., Orsolini, G., Tripi, G., Viapiana, O., Idolazzi, L., Zamò, A., Bonadonna, P., Kunnathully, V., Adami, S., & Gatti, D. (2016). Prevalence, pathogenesis, and treatment options for mastocytosis-related osteoporosis. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 27(8), 2411–2421.
- 6. Theoharis C. Theoharides, William Boucher, Kathleen Spear; Serum Interleukin-6 Reflects Disease Severity and Osteoporosis in Mastocytosis Patients. Int Arch Allergy Immunol 1 August 2002; 128 (4): 344–350.
- 7. Biosse-Duplan, M.; Baroukh, B.; Dy, M.; de Vernejoul, M.C.; Saffar, J.L. Histamine promotes osteoclastogenesis through the differential expression of histamine receptors on osteoclasts and osteoblasts. Am. J. Pathol. 2009, 174, 1426–1434.
- 8. Rossini, M.; Viapiana, O.; Zanotti, R.; Tripi, G.; Perbellini, O.; Idolazzi, L.; Bonifacio, M.; Adami, S.; Gatti, D. Dickkopf-1 and sclerostin serum levels in patients with systemic mastocytosis. Calcif. Tissue Int. 2015, 96, 410–416.
- 9. Rabenhorst A, Christopeit B, Leja S, Gerbaulet A, Kleiner S, Forster A, Raap U, Wickenauser C, Hartmann K (2013) Serum levels of bone cytokines are increased in indolent systemic mastocytosis associated with osteopenia or osteoporosis. J Allergy Clin Immunol 132:1234–1237
- 10. Zhu, J. J., Mahendran, D., Lee, M. H., Seah, J., Fourlanos, S., Varadarajan, S., Ghasem-Zadeh, A., MacIsaac, R. J., & Seeman, E. (2018). Systemic mastocytosis identified in two women developing fragility fractures during lactation. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 29(7), 1671–1674
- 11. Onnes, M. C., van Doormaal, J. J., van der Veer, E., Versluijs, J. B., Arends, S., & Oude Elberink, H. N. G. (2020). Fracture Risk Reduction by Bisphosphonates in Mastocytosis?. The journal of allergy and clinical immunology. In practice, 8(10), 3557–3564.
- 12. Orsolini, G., Gavioli, I., Tripi, G., Viapiana, O., Gatti, D., Idolazzi, L., Zanotti, R., & Rossini, M. (2017). Denosumab for the Treatment of Mastocytosis-Related Osteoporosis: A Case Series. Calcified tissue international, 100(6), 595–598.
- 13. Zhang, L., Wang, T., Chang, M., Kaiser, C., Kim, J. D., Wu, T., Cao, X., Zhang, X., & Schwarz, E. M. (2017). Teriparatide Treatment Improves Bone Defect Healing Via Anabolic Effects on New Bone Formation and Non-Anabolic Effects on Inhibition of Mast Cells in a Murine Cranial Window Model. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research, 32(9), 1870–1883.
- 14. Patlas, N., Golomb, G., Yaffe, P., Pinto, T., Breuer, E., & Ornoy, A. (1999). Transplacental effects of bisphosphonates on fetal skeletal ossification and mineralization in rats. Teratology, 60(2), 68–73.
- 15. Graepel, P., Bentley, P., Fritz, H., Miyamoto, M., & Slater, S. R. (1992). Reproduction toxicity studies with pamidronate. Arzneimittel-Forschung, 42(5), 654–667.
- 16. Sokal, A., Elefant, E., Leturcq, T., Beghin, D., Mariette, X., & Seror, R. (2019). Pregnancy and newborn outcomes after exposure to bisphosphonates: a case-control study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 30(1), 221–229.
- 17. Levy, S., Fayez, I., Taguchi, N., Han, J. Y., Aiello, J., Matsui, D., Moretti, M., Koren, G., & Ito, S. (2009). Pregnancy outcome following in utero exposure to bisphosphonates. Bone, 44(3), 428–430.